BioNTech has signed an agreement to buy a new manufacturing site in Germany from Novartis AG in a move to ramp up Covid-19 vaccine production capacity by up to 750 million doses per year.
BioNTech (BNTX) announced that it has signed a share purchase agreement with Novartis for the site, which will help its plans to be able to produce up to 250 million doses of the Covid-19 vaccine candidate BNT162b2 in the first half of 2021.The transaction is expected to close in the fourth quarter of 2020. Financial terms of the deal weren’t disclosed. BioNTech and Pfizer are testing the BNT162b2 candidate in a global Phase 3 trial.
The German facility is slated to start the production of so-called messenger RNA (mRNA) technology for a COVID-19 vaccine in the first half of 2021, pending regulatory approval.
“This acquisition reflects BioNTech’s commitment to significantly expanding its manufacturing capacity in order to supply a potential vaccine worldwide upon authorization or approval,” said BioNTech CFO Sierk Poetting. “We are working closely with Novartis to prepare for a smooth transition. From a strategic standpoint, the new site will bolster our vertically integrated business model with in-house manufacturing capabilities for mRNA manufacturing as well as vaccine formulation.”
Assuming clinical success, Pfizer (PFE) and BioNTech, plan to seek regulatory review for BNT162b2 as early as October 2020. Previously, the companies had said that, if regulatory approval is obtained, they would be seeking to supply up to 100 million doses worldwide by the end of 2020 and approximately 1.3 billion doses by the end of 2021.
The Marburg-based production site is a multi-platform GMP certified manufacturing facility that currently employs 300 people. It is fully equipped for the production of recombinant proteins as well as cell and gene therapies and has cell culture labs and viral vector production capacities, BioNtech said. The facility is expected to operate as one of the largest mRNA manufacturing sites in Europe alongside 2 of BioNTech’s existing GMP facilities which currently produce the COVID-19 vaccine candidates for clinical trials, and in addition to at least 4 Pfizer production sites in the US and Europe.
The company added that the site acquisition will boost commercial manufacturing capacity to produce its mRNA COVID-19 vaccine candidate BNT162. The BNT162 program includes 5 mRNA vaccine candidates currently in clinical testing in the US, Europe, South America, and China.
In addition, BioNTech plans to manufacture other therapeutic and vaccine drug candidates at the plant, such as mRNA vaccines, antibody, and cell and gene therapy product candidates to support the development of its diversified cancer and infectious disease product pipeline.
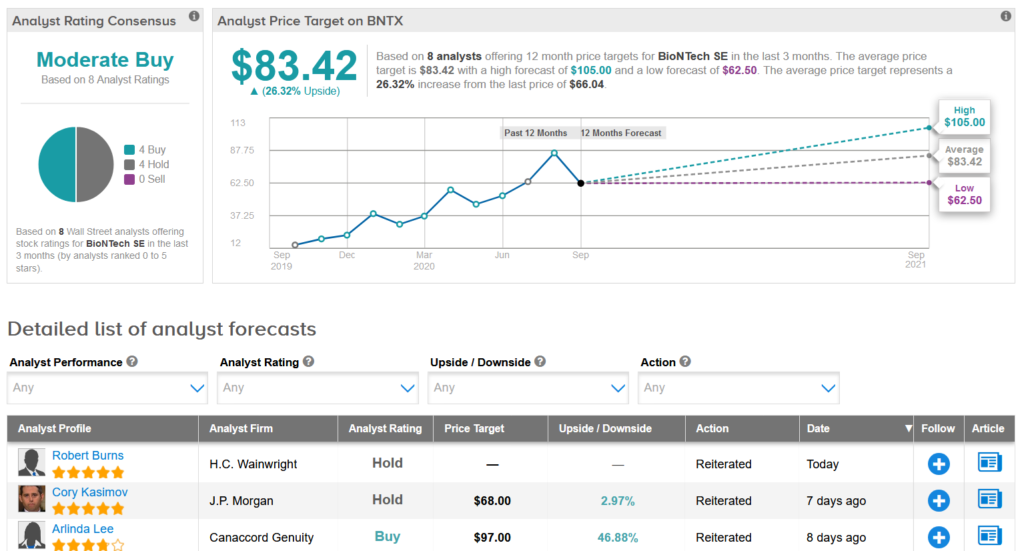
Shares in BioNTech have spiked 95% so far this year and analysts forecast another 26% upside potential setting the average price target at $83.42.
Meanwhile, H.C. Wainwright analyst Robert Burns reiterated a Hold rating on the stock.
“Despite the encouraging preclinical and clinical results seen for BNT162b2 thus far, we cannot predict the vaccine’s commercial potential with certainty, given the continually evolving epidemiology of COVID-19, and the rapidly changing nature of the competitive landscape,” Burns wrote in a note to investors.
The rest of the Street is cautiously optimistic on the stock with a Moderate Buy analyst consensus. (See BioNTech stock analysis on TipRanks).
Related News:
Sorrento Pops 28% On FDA Nod To Kick Off Covid-19 Antibody Trial
Novavax Inks Deal With India For 2B Covid-19 Vaccine Doses In 2021
Merck Puts Focus On Lower Debt, Sees Smaller Takeovers After 2022

