Mesoblast’s (MESO) shares are cooling off today. After skyrocketing 140% on Friday, the Australian biotech fell 25% today.
The lift off came following the release of strong preliminary data concerning its stem cell candidate for treating COVID-19 patients.
Mesoblast’s allogeneic mesenchymal stem cell candidate remestemcel-L showed exceptional promise when given to 12 ventilator dependent COVID-19 patients with acute respiratory distress syndrome (ARDS).
The 12 patients were given two intravenous infusions of remestemcel-L and within the first five days, ten patients (83%) survived, while nine patients (75%) were removed off ventilator support after a median follow up of ten days. Seven of the patients (58%) were discharged from the hospital.
Although a 12 people study is rather small, to put the results into context, in a large study consisting of 5700 patients of ventilator dependent COVID-19 patients, conducted at another New York hospital, the mortality rate was 88%.
Additionally, only 9% of COVID-19 patients who received standard-of-care treatment in another hospital, were able to come off ventilator support in a 4103-patient study published in medRxiv in early April.
H.C. Wainwright’s Swayampakula Ramakanth believes the early results are encouraging and indicate promise for the success of Mesoblast’s Phase 2/3 study of remestemcel-L in patients with COVID-19 associated ARDS. The study should commence shortly, with enrollment expected to be finalized over the next 3 months. The trial will have the 28-day mortality rate as its primary endpoint.
“We are encouraged by these early results and believe that it bodes well for the success of Mesoblast’s Phase 2/3 study of remestemcel-L in patients with COVID-19 associated ARDS. Recall, the Phase 2/3 study is expected to be a 1:1 randomized, placebocontrolled trial in 240 COVID-19 patients with ARDS with a primary endpoint of 28-day mortality rate. Secondary endpoints could include safety, days without ventilator, and survival at different time points. The study is expected to initiate imminently, and management expects to complete patient enrollment in three months. An interim readout is expected at the completion of enrollment of one third of the planned study population. We believe the use of remestemcel-L for the treatment of ARDS could become a significant upside for the company,” said Ramakanth.
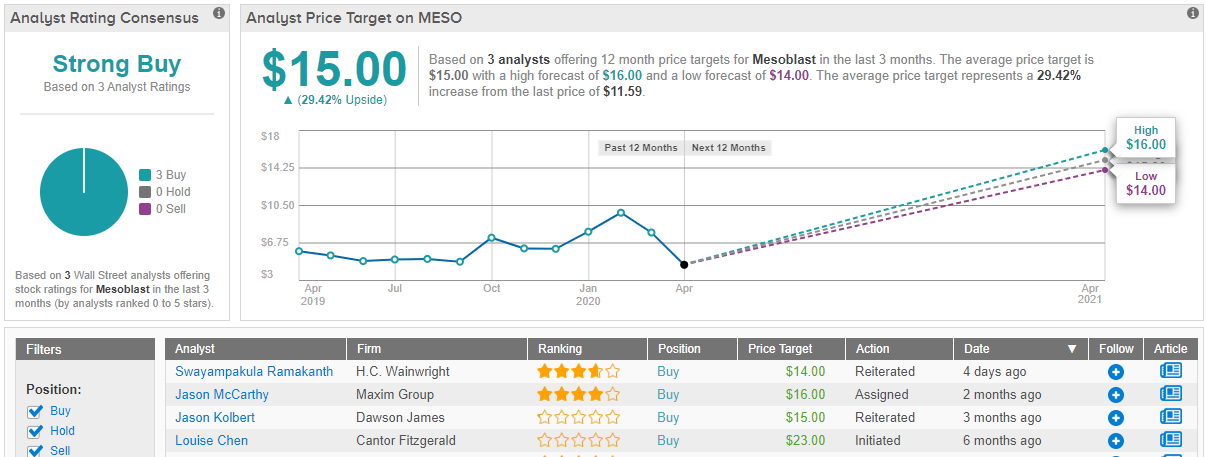
Ramakanth reiterated a Buy on Mesoblast along with a $14 price target, which implies a potential upside of 21% from current levels. (To Ramakanth’s track record, click here)
Only two fellow analysts have published reviews of Mesoblast over the last 3 months, both recommending the stock a Buy. Accordingly, MESO has a Strong Buy consensus rating. The average price target, at $15.00, is a bit more bullish than Ramakanth’s – a 29% for Mesoblast shares. (See Mesoblast stock analysis on TipRanks)
To find good ideas for healthcare stocks trading at attractive valuations, visit TipRanks’ Best Stocks to Buy, a newly launched tool that unites all of TipRanks’ equity insights.