Guido Rasi, the Executive Director of the European Medicines Agency (EMA), has suggested that Gilead’s (GILD) Covid-19 treatment remdesivir could be conditionally approved in Europe as soon as this week.
“It might be that a conditional market authorization can be issued in the coming days,” Rasi revealed to an EU Parliament hearing in Brussels on May 18.
Conditional marketing authorizations are valid for one year and can be renewed annually. They are granted by the EMA for medicines where the benefit of immediate availability outweighs the risk of less comprehensive data than normally required.
Indeed, remdesivir has already received the green light from the EMA for compassionate use for patients during this pre-authorization phase.
GILD also received an Emergency Use Authorization (EUA) from the United States for the drug on May 1.
Since then Gilead has signed non-exclusive licensing agreements with five generic pharmaceutical manufacturers to produce and sell remdesivir in 127 countries, including India and Pakistan.
Nonetheless JP Morgan’s Cory Kasimov recently maintained his Hold rating on the stock with an $85 price target. Given the stock’s 17% year-to-date climb, the analyst’s price target indicates upside potential of 11% from current levels.
“Although we’re impressed by the company’s intensive work with remdesivir, we believe that GILD received ample market credit for it and remdesivir is unlikely to result in tangible long-term cash flows,” Kasimov told investors. “A short-term $5 billion boost sales has marginal impact on our overall valuation and other opportunities for growth will take time to play out.”
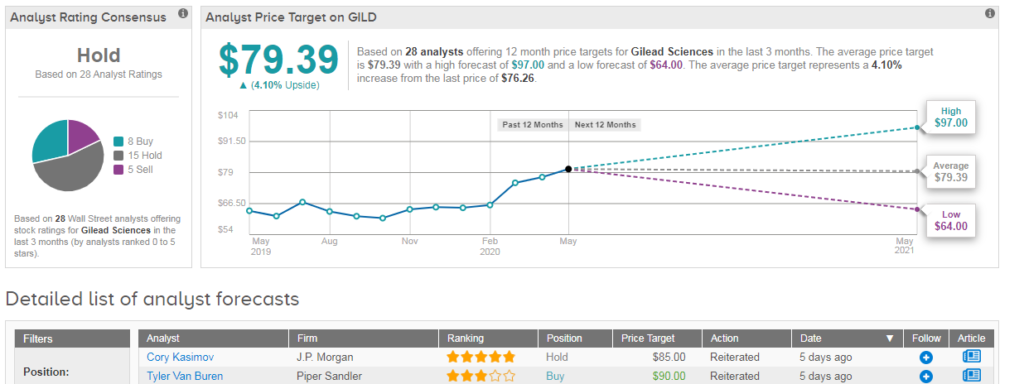
Overall TipRanks data shows that out of the 28 analysts covering Gilead in the past three months, 15 have a Hold rating on the stock, 8 say Buy and 5 say Sell, adding up to a Hold consensus. The $79.39 average price target suggests 4% upside potential lies ahead. (See Gilead stock analysis on TipRanks)
Related News:
Gilead Signs Remdesivir Licensing Agreements With Five Drugmakers
AstraZeneca Aiming For 30M UK Covid-19 Vaccine Doses By September
Pfizer Plans To Test Covid-19 Vaccine On Thousands Of Patients By September- Report

