The majority of a small group of coronavirus patients showed their condition improve after treatment with remdesivir, an experimental drug being developed by Gilead Sciences Inc. (GILD), according to a study published in the The New England Journal of Medicine.
The study is based on results from a cohort analysis of 53 patients hospitalized with severe COVID-19 symptoms in the U.S. and around the world, who were treated with the investigational antiviral remdesivir on an individual compassionate use basis. About two-thirds of the patients showed improvement in oxygen support and 17 of 30 of those treated who were on ventilators were able to be taken off the life-support machines. Moreover almost half of the treated patients were discharged from hospital.
“We cannot draw definitive conclusions from these data, but the observations from this group of hospitalized patients who received remdesivir are hopeful,” said Jonathan D. Grein, Director of Hospital Epidemiology, Cedars-Sinai Medical Center, Los Angeles, and lead author of the journal article. “We look forward to the results of controlled clinical trials to potentially validate these findings.”
Remdesivir is an investigational drug that has not been approved by any regulatory authority, and the safety and efficacy of remdesivir for the treatment of COVID-19 are not yet known. Remdesivir was originally developed for the Ebola virus by Gilead. More recently, studies have shown it could potentially block the ability of SARS-CoV-2, the virus that causes COVID-19, to replicate.
“While the outcomes observed in this compassionate use analysis are encouraging, the data are limited,” said Merdad Parsey, Gilead’s Chief Medical Officer. “Gilead has multiple clinical trials underway for remdesivir with initial data expected in the coming weeks.”
Gilead’s CEO Daniel O’Day said Friday that the company expected to have preliminary data from remdesivir study in severe patients at the end of April. O’Day disclosed that while data publication from the China remdesivir trials was with Chinese investigators, the study in patients with severe symptoms was stopped due to stalled enrolment. O’ Day added that in May the company anticipated to get initial data from the placebo-controlled NIAID trial as well as data from the Gilead study of patients with moderate symptoms of COVID-19.
Gilead shares have risen about 13% this year fueled by prospects that its remdesivir experimental drug might have the best shot at becoming a treatment for the coronavirus.
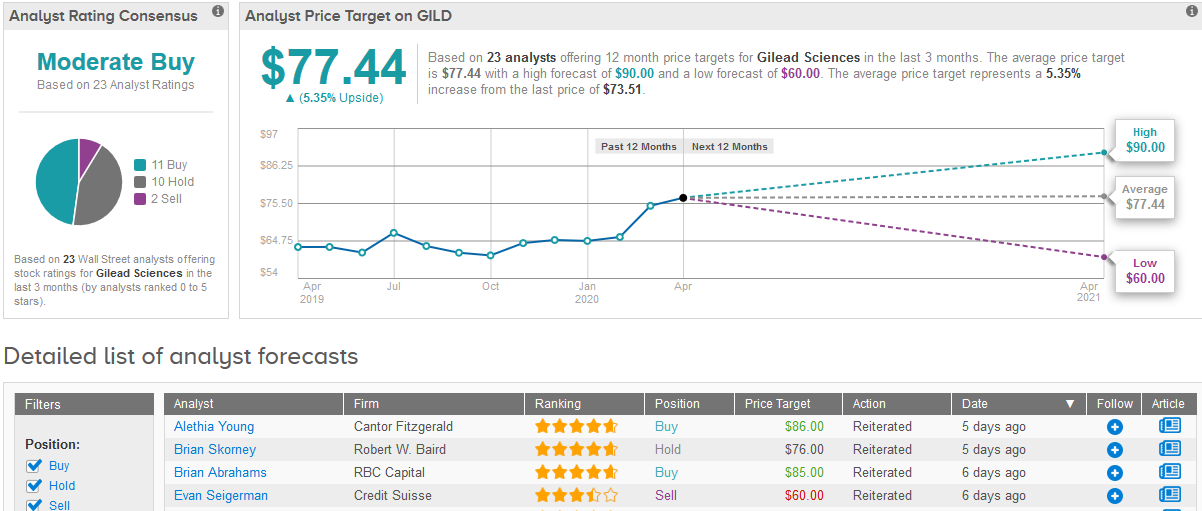
TipRanks analytics show that out of the 23 analysts covering the stock in the past three months, 11 are bullish with a Buy rating on Gilead stock, 10 are sidelined with a Hold rating and 2 are bearish with a Sell rating, adding up to a Moderate Buy consensus rating. The 12-month average price target of $77.44 projects a slight 5.4% upside potential.
Gilead boasts a 10 score from TipRanks Smart Score. That’s thanks to a combination of bullish datapoints, including a bullish positive sentiment from investors, increased hedge fund activity, positive news sentiment and even bullish opinions from the financial blogging community. (See Gilead stock analysis on TipRanks)
Related News:
Microsoft’s Bill Gates to Spend Billions of Dollars on Coronavirus Vaccine Development
Google, Apple Join Forces to Develop Coronavirus Tracking Technology
Chembio Spikes 8% As Covid-19 Antibody Test Picked For Study