Moderna Inc. (MRNA) announced on Tuesday that its experimental coronavirus vaccine has been granted fast track status by the U.S. Food and Drug Administration (FDA).
The fast track status designation is awarded to speed up the regulatory review of its novel coronavirus vaccine candidate (mRNA-1273).
Last week, the FDA completed its review of Moderna’s Investigational New Drug (IND) application for mRNA-1273 allowing it to proceed to a Phase 2 study, which is expected to begin shortly. The clinical stage biotech company said it is finalizing the protocol for a Phase 3 study, expected to begin in early summer of 2020.
“Fast Track designation underscores the urgent need for a vaccine against the novel coronavirus,” said Tal Zaks, Chief Medical Officer at Moderna. “As we await the full set of clinical data from the NIAID-led Phase 1 study, we are actively preparing for our Phase 2 and Phase 3 clinical studies to continue learning about the potential of mRNA-1273 to protect against SARS-CoV-2.”
By utilizing messenger RNA (mRNA) science, Moderna hopes to create a new class of transformative medicines for patients. mRNA medicines are designed to direct the body’s cells to produce intracellular, membrane or secreted proteins that can have a therapeutic or preventive benefit and have the potential to address a broad spectrum of diseases.
Moderna shares dropped 2.7% to $65.04 in morning U.S. trading after the stock more than tripled this year.
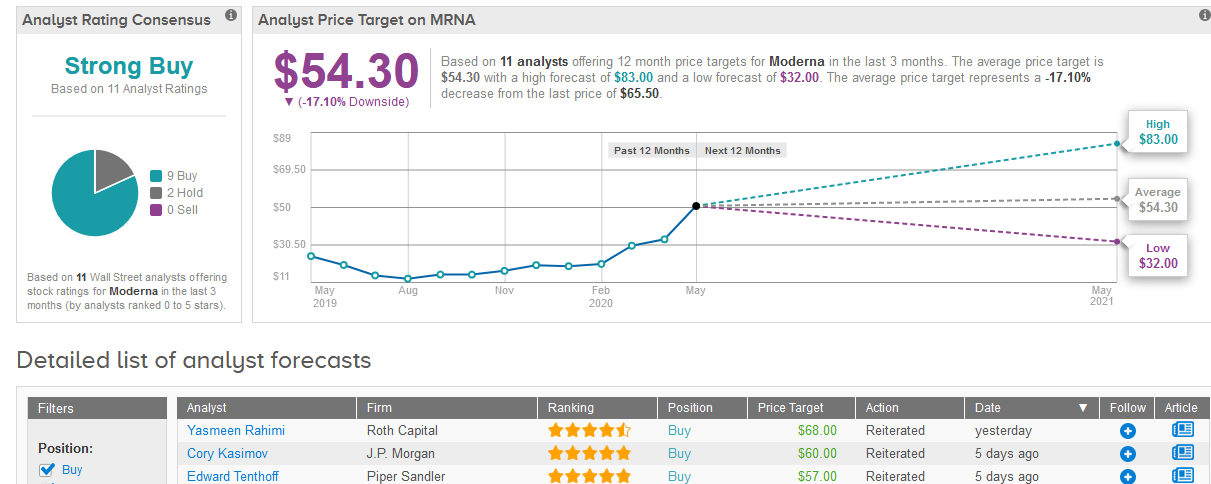
Five-star analyst Yasmeen Rahimi at Roth Capital raised Moderna’s price target to $68 from $41, while maintaining a Buy rating on the shares, saying that with the near-term start of a Phase 2 study for its COVID-19 vaccine, the company remains the front-runner in getting a vaccine to pivotal trials with potential approval by 2021.
Although analysts have a Strong Buy consensus rating on Moderna stock based on 9 Buys and 2 Holds, its recent rally means that the $54.30 average price target now indicates 17% downside potential from current levels. (See Moderna stock analysis on TipRanks).
Related News:
Novavax Spikes 31% on $384 Million Cash Injection for Vaccine Production
CymaBay Doubles After-Hours On Positive NASH Trial Update
Genfit Craters 49% On Failed NASH Trial; Top Analyst ‘Not Surprised’