Shares of Novavax popped almost 23% in Thursday’s extended market session after the biotech company announced that its coronavirus vaccine candidate showed to be 96.4% effective against the original COVID-19 strain in a late-stage trial in the UK.
Additionally, the results of the Phase 3 trial demonstrated that Novavax’s (NVAX) protein-based COVID-19 vaccine candidate, NVX‑CoV2373, was 86.3% effective in protecting against the B.1.1.7/501Y.V1 virus variant first found in the UK. According to a complete analysis of the drugmaker’s Phase 2b trial conducted in South Africa, NVX‑CoV2373 showed an efficacy of 55.4% among HIV- negative trial participants in a region where the vast majority of strains are B1.351 escape variants. In both, the UK and South Africa trials, the vaccine was well-tolerated with no cases of severe illness or deaths among participants.
The data from both studies, which built on interim results announced in January 2021, are expected to serve as the basis for submission for authorization of the vaccine to regulatory agencies worldwide, the company said.
“We are very encouraged by the data showing that NVX-CoV2373 not only provided complete protection against the most severe forms of disease, but also dramatically reduced mild and moderate disease across both trials. Importantly, both studies confirmed efficacy against the variant strains,” commented Novavax CEO Stanley C. Erck. “Today marks one year since the WHO officially declared the COVID-19 pandemic, and with this data in hand, we are even more motivated to advance our vaccine as a potential weapon in the fight to end the suffering caused by COVID-19.”
NVX‑CoV2373 is a stable, prefusion protein made using Novavax’s recombinant protein nanoparticle technology and includes the company’s proprietary Matrix‑M adjuvant. The purified protein is encoded by the genetic sequence of the SARS-CoV-2 spike (S) protein and is produced in insect cells. It is stable at 2°C to 8°C and is shipped in a ready-to-use liquid formulation that allows distribution using standard vaccine supply chain channels.
The UK trial enrolled more than 15,000 participants between 18-84 years of age, including 27% over the age of 65. The South Africa trial included 2,665 healthy adults and 240 medically stable, HIV-positive adults.
NVAX shares have plunged 41% over the past month, but are still up 68% on a year-to-date basis. (See Novavax stock analysis on TipRanks).
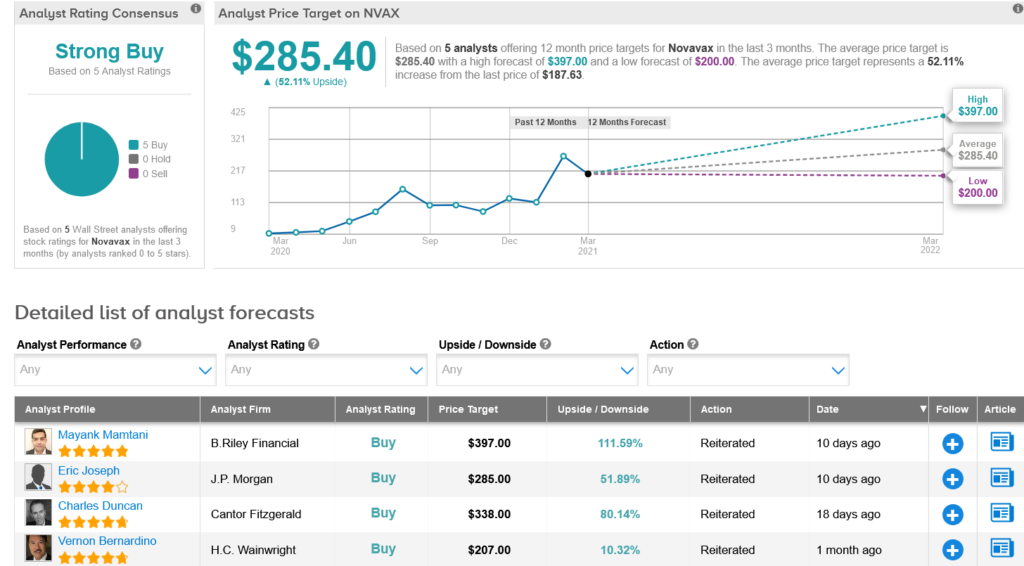
Earlier this month, B. Riley Financial analyst Mayank Mamtani reiterated a Buy rating on the stock with a Street-high $397 price target (112% upside potential), citing NVAX’s ongoing effort to explore the potential of a combo vaccine, which would include NanoFlu and NVX-CoV2373.
This “bodes well in the post-pandemic setting, where NVAX remains the only vaccine maker possessing near-commercial influenza and C-19 vaccine programs,” Mamtani wrote in a note to investors. “We note increased pace of vaccinations, anticipated to improve further by recent EUA of JNJ’s C-19 vaccine, and declining US case count, could maintain continued stock volatility, which we view as an additional opportunity to accumulate NVAX shares.”
The rest of the Street is firmly in line with Mamtani’s bullish outlook. The Strong Buy consensus rating is based on 5 unanimous Buy ratings. That’s alongside an average analyst price target of $285.40, which indicates 51% upside potential from current levels.
Related News:
DocuSign’s 4Q Results Beat Analysts’ Expectations; Shares Dip 4%
Cloudera Drops 8% On Disappointing FY22 Outlook
Redwood Trust Ramps Up Dividend By 14%; Street Is Bullish

