U.S. drugmaker Pfizer Inc. (PFE) and BioNTech SE (BNTX) advanced after announcing that they have initiated U.S. clinical human testing trials of their potential coronavirus vaccine candidates.
First participants have been dosed in the U.S. in the Phase 1/2 clinical trial for the BNT162 vaccine program to prevent COVID-19 after the dosing of the first cohort in Germany was completed last week, the companies said in a joint statement. Pfizer shares rose 3% to $38.75 and German partner BioNtech soared $6.6% to $48.81 in midday U.S. trading.
“The short, less than four-month timeframe in which we’ve been able to move from pre-clinical studies to human testing is extraordinary and further demonstrates our commitment to dedicating our best-in-class resources, from the lab to manufacturing and beyond, in the battle against COVID-19,” said Pfizer Chairman and CEO Albert Bourla.
If the clinical trials for the experimental vaccine show to be safe and effective, Pfizer and BioNTech will be ready to ramp up production for global supply. Pfizer said it plans to activate its extensive manufacturing network to produce an approved COVID-19 vaccine as quickly as possible. This should allow for distribution of millions of vaccine doses as early as this year, increasing to hundreds of millions in 2021.
Pfizer’s joint program includes four vaccine candidates, each representing a different combination of messenger RNA (mRNA) format and target antigen.The Phase 1/2 study is designed to determine the safety, immunogenicity and optimal dose level of four mRNA vaccine candidates evaluated in a single, continuous study. In the first stage of the Phase 1/2 trial in the U.S., 360 healthy individuals will be enrolled into two age cohorts (18-55 and 65-85 years of age).
If the vaccine receives regulatory approval, BioNTech and Pfizer will jointly commercialize the vaccine on a global scale – except for China, where BioNTech has a collaboration with Fosun Pharma.
Five-star analyst Robert Burns at H. C. Wainwright reiterated his Hold rating on BioNTech’s stock with a $48 price target, “in anticipation of direct evidence that BNT162 can elicit a meaningful antibody response against SARS-CoV-2, the pathogen that causes COVID-19, along with data demonstrating the vaccine’s protective effect”.
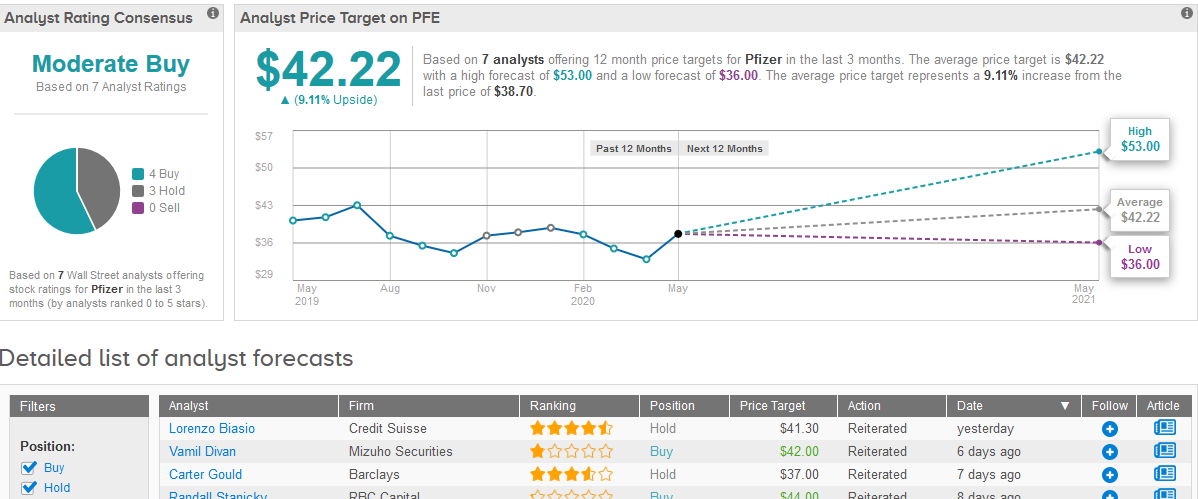
Overall, Wall Street analysts have a Moderate Buy consensus rating for both Pfizer and BioNtech. For Pfizer, ratings are divided between 4 Buys and 3 Holds and for BioNtech they are evenly split between 2 Buys and 2 Holds.
The $42.22 average price target for Pfizer projects 9% upside potential for the drugmaker’s shares in the coming 12 months. (See Pfizer stock analysis on TipRanks).
While for BioNTech, analysts forecast 7.4% downside potential setting the average price target at $45.25. (See BioNTech stock analysis on TipRanks).
Related News:
Amarin (AMRN) Stock Could Stage a Rebound to $15, Says Analyst
3 Biotech Stocks With Plenty of Upside in the Pipeline
Roche Gets Emergency FDA Approval For Covid-19 Antibody Test, Starts Shipping