Shares in Zynerba Pharmaceuticals (ZYNE) plunged 48% on Tuesday after the company announced disappointing top line results from the 14-week pivotal CONNECT-FX trial. The stock is now rallying 10% in Wednesday’s pre-market trading.
The multi-national, randomized, double-blind, placebo-controlled trial assessed the efficacy and safety of Zygel CBD gel as a treatment for behavioral symptoms of Fragile X syndrome (FXS) in 212 patients.
Zygel did not achieve statistical significance versus placebo in the primary endpoint of improvement in the Social Avoidance subscale of the Aberrant Behavior Checklist – Community FXS. Zygel also did not demonstrate statistical significance versus placebo in the three key secondary endpoints, namely Irritability, Socially Unresponsive/Lethargic and Clinical Global Impression.
However, a pre-planned ad hoc analysis of the most severely impacted patients in the trial, (with at least 90% methylation of the impacted FMR1 gene), demonstrated statistical significance in the primary endpoint of improvement at 12 weeks of treatment in the Social Avoidance subscale compared to placebo (p=0.020).
This group comprised 80% of the patients enrolled in the CONNECT-FX study- and occurs in approximately 60% of the overall FXS patient population. Based on this analysis, Zynerba intends to meet with the FDA as soon as possible regarding a regulatory path forward for Zygel.
“This study identified a key population of patients who appear to benefit from treatment of their behavioral symptoms of FXS with Zygel,” said Randi J. Hagerman, an investigator in the clinical trial. “Zygel has the potential to be an important therapeutic option for the most severely impacted patients with Fragile X” he added.
Zygel was very well tolerated in CONNECT- FX, and the safety profile was consistent with previously released data from other Zygel clinical trials.
Fragile X syndrome is a rare genetic developmental disability that is the leading known cause of both inherited intellectual disability and autism spectrum disorder, affecting 1 in 3,600 to 4,000 males and 1 in 4,000 to 6,000 females. In the US, there are about 71,000 people suffering with FXS.
Following the news Needham analyst Serge Belanger took a more bearish approach on the stock, writing “We are downgrading ZYNE to Hold based on Zygel missing its endpoints in its first placebo-controlled FXS trial and the uncertainty regarding the proposed path forward of the program.”
Belanger is skeptical that the FDA will agree to ZYNE’s proposed path to Zygel approval without requiring an additional trial that could take 2 years to complete. “The clinical data supporting Zygel’s efficacy in FXS is thin given that the CONNECT-FX trial missed all endpoints and the ad hoc subset analysis results, while positive for the primary endpoint, missed on secondary endpoints” he notes.
And indeed, until now, Zygel has reported results from three open-label phase 2 trials in three different indications (FXS, DEE, and ASD) but has yet to demonstrate efficacy in a placebo-controlled trial. “Investors are likely to remain skeptical of Zygel’s efficacy and potential until it is validated by positive results from a placebo-controlled trial” the analyst concludes.
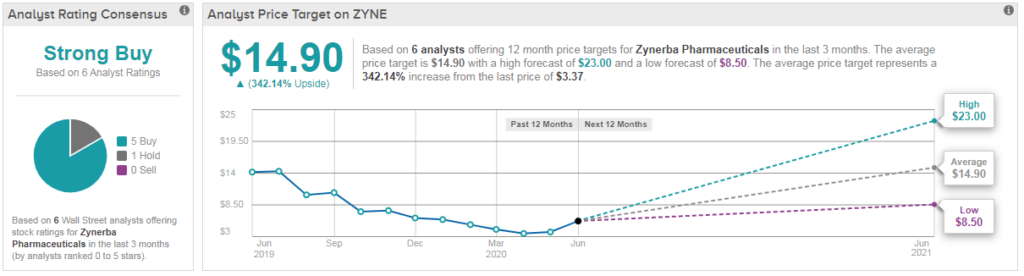
Nonetheless the stock retains its Strong Buy analyst consensus with a $15 average analyst price target. Michael Higgins of Ladenberg Thalmann reiterated his buy rating on July 1, but slashed his price target to $10 from $26 previously.
“For now, we look for a confirmatory Phase 3 to be run, with a launch in 2023 treating the 60% with full mutation, a slightly lower cost (unless approved as a seizure medication prior), but continued penetration rates in those most severe” the analyst wrote. (See ZYNE stock analysis on TipRanks)
Related News:
Akero Spikes 42%- And Continues To Rally After-Hours- On New NASH Data
CRISPR Therapeutics Prices Public Offering; Analysts Bullish On CTX130 Potential
Gilead Sets Pricing for Covid-19 Treatment Remdesivir at $2,340 Per Patient

